News & Media
eSUNMed Preparation and Application of PCL Micro-powder
2022-10-27
At present, cosmetic injection has become more and more popular. Its simple operation, minimal trauma, rapid recovery, low infection rate and good curative effect make it more and more widely used in cosmetic.
The filling injections used in the industry mainly include hyaluronic acid, collagen, regeneration and other materials. In the past, hyaluronic acid was the main filling material in China. Since 2021, products based on recombinant collagen and recycled materials have been approved successively. Regenerative injection is a new growth point of the medical and aesthetic injection market in China. Regenerative injection can stimulate human collagen regeneration to achieve filling effect through PLLA and PCL micropowders. It has the advantages of “safer, more natural, more long-term, mass production”, and is a high-end upgrading filling material.
Hyaluronic acid and biomedical materials PLA/PCL
From the ordinary familiar “hyaluronic acid” to the regenerative injection, What are the characteristics of these different materials?
1.Hyaluronic Acid
Hyaluronic acid is a kind of polysaccharide with chain structure. Different molecular weight and cross-linking degree can present different properties, meeting different medical and aesthetic demands. Due to the relatively simple molecular structure, the current molecular weight control and cross-linking degree control technology of hyaluronic acid is relatively mature. Due to its balanced performance in security, filling effect and durability, and high cost performance, it still occupies the mainstream of the market.
2.Regenerative injection:PLA/PCL
The main components of the regeneration injection are PLLA (Poly-L-L-lactic Acid) or PCL (Polycaprolactone), both of which are synthetic polymer materials, which can achieve mass production in a large scale. At the same time, the micropowders preparation technology of materials is constantly developing, and medical and aesthetic manufacturers can control the diameter of PLLA/PCL micropowders to 20-70, which is suitable for human injection and metabolism μ m; With the continuous development of suspension technology, some products can realize the combination of PLLA/PCL and other gel materials.
The progress of production technology has gradually expanded the application scenario of renewable materials from the medical end to the medical and aesthetic end, and promoted the enrichment, efficacy optimization and market recognition of medical and aesthetic injection products. Regenerative injection can stimulate the regeneration of collagen, with good long-term effect and naturalness, and its price is high, focusing on high-end market.
Difference of PLA/PCL injection
At present, the popular beauty items on the market, Maiden Needle (using PCL material) and Tongyan Needle (using PLA material) have their own advantages and can be used in different regions.
Girls needle and children’s face needle have their own advantages. The former has a quick effect, while the latter has a gradual effect. The effect will be significant after 3-6 months. If you want to pursue the true nature, the girl needle is a more appropriate choice; However, if you want to pursue lasting effects, Tongyan Needle is even better.
eSUNMed Preparation and Application of PCL Micropowders
Last article, we introduced the preparation of eSUNMed (lactic acid) PLLA micropowders and their application in the medical and aesthetic industry. This article will focus on PCL micropowders.
The preparation of micropowder requires uniform and controllable particle size. Its particle size is uneven, which may cause “complex effects during use”, “increased side effects of drug products” and other drawbacks. This puts forward corresponding requirements for raw materials and preparation technology.
1.Introduction to PCL Materials
Polycaprolactone is produced by ε- Caprolactone is a kind of degradable synthetic polymer material formed by ring opening polymerization catalyzed by metal anion complex catalyst. It is insoluble in water, but soluble in a variety of organic solvents; In the natural environment, it can be completely degraded into carbon dioxide and water, and the degradation products can be absorbed or eliminated by the body. Good biocompatibility makes it widely used in drug controlled release.

eSUNMed adopts continuous polymerization process to replace batch polymerization, which solves the problem of uneven mass and heat transfer of materials, achieves stable production batches, guarantees the stability of batch materials, and the molecular weight distribution of products is less than 1.6.
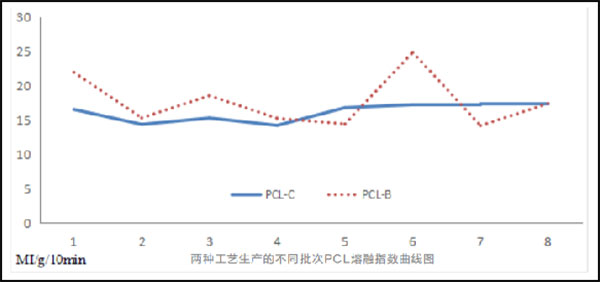
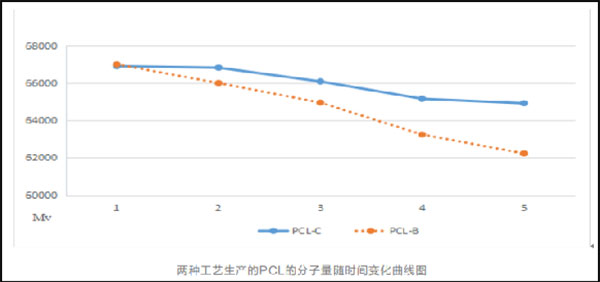
2.Preparation Technology of PCL Micropowders
Emulsification and spray drying methods were used to prepare PCL micropowders. Emulsification is a commonly used method to prepare micropowders, with mature technology, large controllable output, strong plasticity and functionality of products. Through the membrane emulsification technology, the lotion particle size can be uniform and controllable, the emulsion drops are stable (longer storage time), the batch repeatability is good, and the energy consumption in the preparation process is low, and the comprehensive cost is lower than other preparation methods.
The preparation of micropowders by spray drying method is fast and easy to operate, which is more suitable for mass production of micropowders. Spray drying technology is suitable for both heat sensitive and heat resistant drugs. It is a continuous preparation process with unique particle design and formation characteristics. Compared with other methods for preparing micropowders, the spray drying method also has its advantages, which can achieve greater particle size control, obtain unique morphology, higher drug encapsulation efficiency, low cost, simple and practical, less solvent use, no surfactant, and low residue.

PCL 100C Schematic diagram of micropowders size distribution(10X/50X)
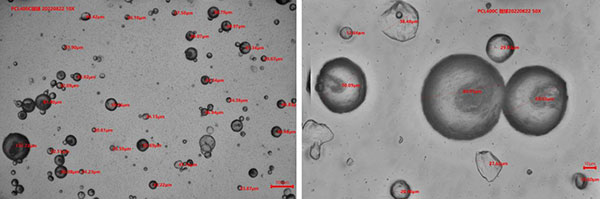
PCL 400C Schematic diagram of micropowders size distribution(10X/50X)
3.Application of PCL Micropowders in the Medical and Aesthetic Direction
Microcrystal polycaprolactone PCL (polycaprolactone) will continue to stimulate the growth of collagen, and the new collagen will gradually replace the CMC gel carrier to form a durable, natural and safe tissue scaffold, which can be maintained for at least one year. Because of the size and surface characteristics of PCL micropowders (completely smooth spheres), they will not be engulfed by macrophages in the human body. Instead, macrophages will form a coating on the surface of PCL micropowders and form a single-layer high-quality collagen scaffold, which can form effective support and elasticity on the face, and have a more immediate, more lasting, and more sustainable therapeutic effect. In addition, PCL will be gradually absorbed and decomposed by the human body after injection, and it is also biocompatible. General users can inject PCL without allergy testing (it does not mean that the human body will not have allergy).
Micropoeders Processing Service
Relying on the advantages of eSUN, its parent company, in the R&D and application of polylactic acid, polycaprolactone and related copolymers over the years, eSUN mainly committed to the development and application of biomedical polymer materials. At present, eSUNMed has mastered the micropowders preparation process and can provide micropowders processing services according to user needs, among which, the micropowders particle size is 3-100 μ m. Conventional molecular weight is less than 100000.


eSUNMed has nearly 400 square meters of 100000 level standard clean workshop and 1 million square meters of 10000 level purification laboratory, meeting the standard GMP workshop for the production of pharmaceutical excipients and sterile medical devices, and meeting the industrial standards for biomedical polymer materials. In addition, we have all kinds of advanced equipment, which can provide stable guarantee for enterprise product research and development, production and application.
If you want to know more about products and related information, please contact us.
Tel:0755-23768972
Address: 3F, Building 9, Yifenghua Innovation Industrial Park, No. 91 of Huaning Road, Dalang Street, Longhua District, Shenzhen


